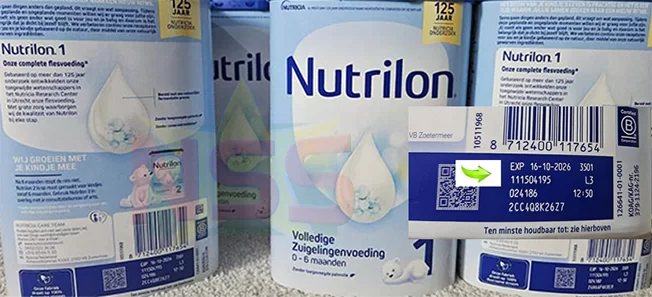
Health authorities across multiple nations have escalated a major recall of Nutrilon infant formula products after discovering potential contamination with cereulide toxin, sparking significant concern among parents in Suriname where the affected batches remain commercially available.
The international recall initiative, originally initiated in European countries including the Netherlands, now encompasses various Nutrilon formulations specifically designed for infants. The products under scrutiny include Nutrilon Stage 1 and specialized AR variants intended for babies with reflux conditions. This regulatory action follows comprehensive evaluations identifying specific batches containing ARA fatty acid sourced from a particular supplier believed to be the contamination origin.
Medical experts warn that cereulide exposure can trigger severe gastrointestinal complications in infants, particularly those under six months of age. Symptoms may include persistent vomiting, diarrhea, and abdominal discomfort, necessitating immediate medical attention if observed.
In Suriname’s Wanica district, one concerned mother shared her apprehension with local media after discovering she possessed contaminated products. She has immediately discontinued usage and initiated contact with both the retail outlet where she purchased the formula and regional suppliers, though official guidance from distributors remains pending at this time.
The Surinamese Ministry of Health has acknowledged the developing situation and is currently conducting its own assessment. Officials have indicated that formal public guidance regarding the recalled products will be issued shortly. Meanwhile, parents are advised to scrutinize batch numbers and expiration dates on all Nutrilon products and refrain from using any items matching the officially recalled batches circulating internationally.