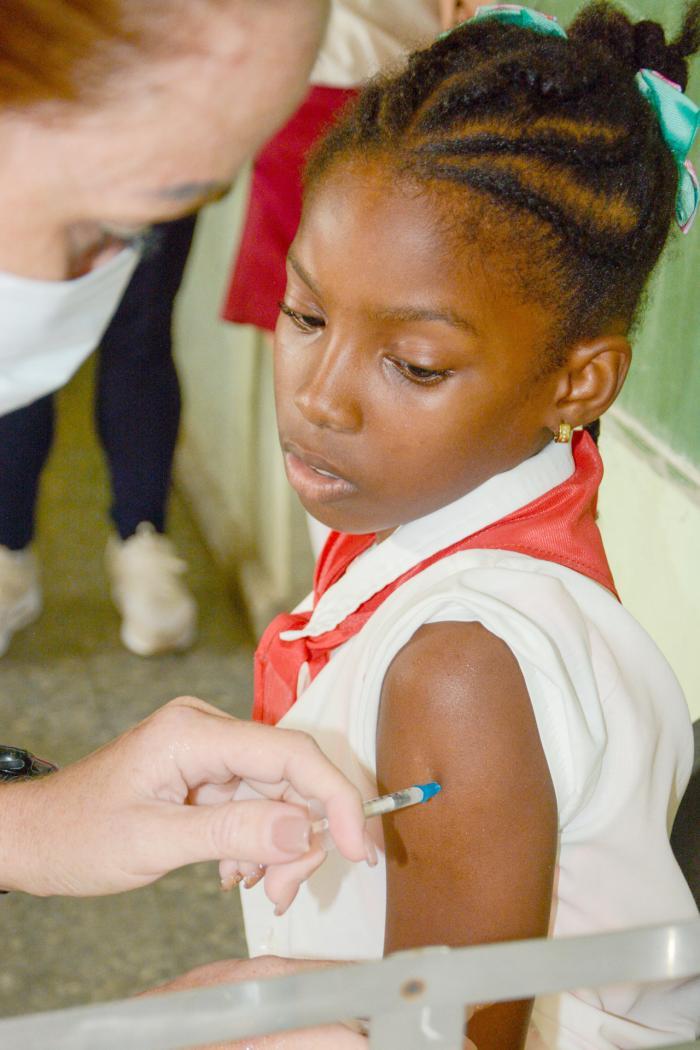
In a significant public health advancement, Cuba has officially integrated the Cecolin HPV vaccine into its national immunization program with formal endorsement from the Pan American Health Organization and World Health Organization (PAHO/WHO). This strategic initiative, launched in October 2025, represents a crucial step toward eliminating cervical cancer through widespread preventive vaccination.
The comprehensive vaccination campaign targets over 68,000 girls across the island nation, specifically focusing on those who have reached nine years of age. The Chinese-manufactured Cecolin vaccine, produced by Innovax, has received WHO prequalification, confirming its safety profile and immunological effectiveness. Medical authorities emphasize that the vaccine generates a more robust immune response than natural infection, ensuring durable protection against high-risk HPV strains.
Health officials have addressed common parental concerns through detailed guidance. The vaccination protocol allows temporary postponement for girls experiencing acute infectious diseases but permits vaccination after recovery from conditions like dengue or chikungunya. The program will be administered through three primary channels: elementary schools with medical personnel present, polyclinic vaccination centers, and certified peripheral facilities in each region.
The selection of nine-year-old recipients reflects scientific evidence demonstrating optimal vaccine effectiveness when administered before sexual debut and potential virus exposure. This approach creates both individual protection and community-wide herd immunity, significantly reducing viral circulation across population demographics.
While the current phase exclusively targets female recipients due to cervical cancer representing approximately 70% of HPV-related cancers, health authorities note that high coverage in girls substantially reduces infection risks for males through indirect protection. The vaccine remains recommended even for individuals with previous HPV exposure, as it prevents future infection with high-risk types 16 and 18 despite not treating existing infections.